X-ray Laser Brings Cellular Messengers into Focus
Last year's Nobel Prize in Chemistry – shared by Stanford School of Medicine Professor Brian Kobilka and Robert Lefkowitz of Duke University – recognized groundbreaking research in G protein-coupled receptors (GPCRs).
By Glenn Roberts Jr.
Last year's Nobel Prize in Chemistry – shared by Stanford School of Medicine Professor Brian Kobilka and Robert Lefkowitz of Duke University – recognized groundbreaking research in G protein-coupled receptors (GPCRs). GPCRs are embedded in cell membranes. They interact with signaling molecules outside of cells and trigger responses within cells. Because of their key role in the body's chemical messaging network, they are also a prime target for developing drugs to treat many diseases; hypertension, asthma, schizophrenia, and Parkinson's disease are among the afflictions associated with GPCR disorders.
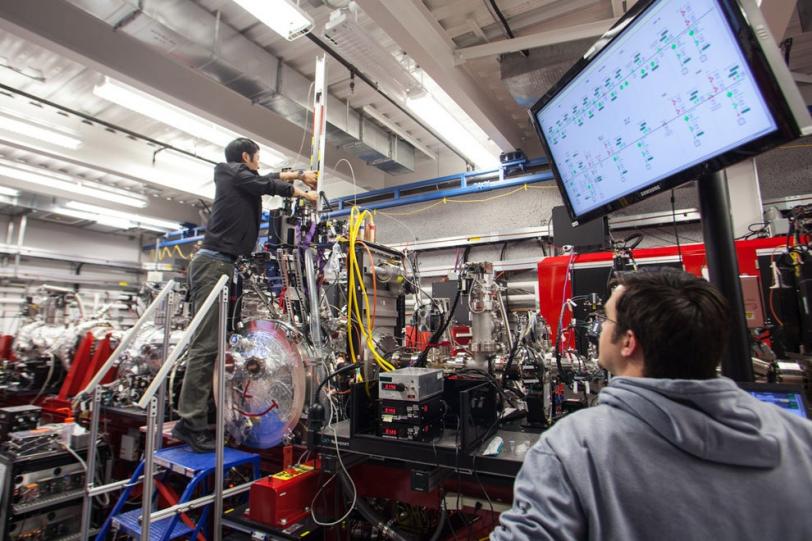
Now, a research team led by Vadim Cherezov of The Scripps Research Institute says preliminary data from a March experiment indicates it has, for the first time, used an X-ray free-electron laser – SLAC's Linac Coherent Light Source – to reconstitute the structure of a G protein-coupled receptor.
This method offers several potential advantages over the methods traditionally used at synchrotron light sources to determine the structures of GPCRs. Experiments at LCLS can be performed on much smaller crystals, just a few microns or less in size, allowing scientists to study more fragile proteins and their complexes that are difficult to crystallize. The short, ultrabright laser pulses allow researchers to gather more information with less radiation damage, with the proteins in a more natural state and at room temperature.
The crystallized receptor samples used in the LCLS experiment were grown and studied in fatty, or lipidic, gel that stabilized them by mimicking their natural environment in a cell membrane.
Cherezov, an assistant professor in the Department of Integrative Structural and Computational Biology at TSRI, worked with other scientists from TSRI and SLAC and collaborators from Arizona State University, Trinity College Dublin in Ireland, the German Electron Synchrotron (DESY) and the Center for Free-Electron Laser Science in Germany in the LCLS experiment.
Describe your recent experiment at SLAC's Linac Coherent Light Source.
CHEREZOV: Our recent experiment at LCLS was focused on collecting diffraction data from tiny microcrystals of GPCRs, grown in a lipidic gel that offers a membrane-like environment. Development of more effective drugs with fewer side effects requires knowledge of the 3-D structure of these receptors and an understanding of the signal and response mechanisms, but GPCRs are very unstable and difficult to crystallize, which makes it challenging to study their structure.
What is unique and pioneering about this LCLS experiment?
CHEREZOV: This was the first attempt to obtain a high-resolution structure of a GPCR at LCLS. We streamed GPCR microcrystals into the path of the X-ray laser pulses inside a gel-like matrix, known as "lipid cubic phase" or LCP, using a specially designed LCP-jet injector. And the experiment was successful, opening the door to future studies of GPCRs and other membrane-protein samples.
How did you become interested in conducting an experiment at LCLS?
CHEREZOV: I learned about LCLS capabilities about two to three years ago from inspiring talks by Petra Fromme and John Spence, both from Arizona State University. At that time they were trying to collect data from tiny crystals of Photosystem I and record images before the crystals were destroyed. These experiments and the whole idea of "diffraction before destruction" seemed to be way ahead of their time – from the realm of science fiction.
Yet, just a few years later, we have a damage-free, room-temperature, high-resolution structure of a GPCR, one of the most difficult to study but biomedically very important families of membrane proteins. This is just mind-boggling and demonstrates the great potential of this unique approach for the future of structural studies.
How does this experiment build upon your previous results?
CHEREZOV: For the last 15 years I was involved in the development of new approaches for membrane protein crystallization in a gel-like state known as "lipidic mesophase." The first application of LCP as a medium for crystallization was introduced in 1996. The method was not widely accepted by the structural biology community at the time, mainly due to the difficulties associated with handling LCP. Now, this method is becoming increasingly popular and is responsible for identifying the structures of about 40 unique membrane proteins from eight different families, and these numbers are growing fast. Seventeen out of 19 published GPCR structures were crystallized in LCP.
What was most challenging about the experiment at LCLS?
CHEREZOV: Our challenges were growing enough microcrystals for the LCLS experiment (Wei Liu, a staff scientist in my group, worked on crystallization protocols), developing an LCP injector (accomplished by Uwe Weierstall and John Spence), preventing the LCP solution from dehydrating (Martin Caffrey's group developed a special lipid to solve this problem) and data processing (performed by Henry Chapman's group).
What was most surprising or interesting about the preliminary data collected at LCLS?
CHEREZOV: One of the most surprising was to see the clarity of data obtained at LCLS in comparison to synchrotron data using much larger crystals. It is gratifying to see that we can obtain better data from smaller crystals here at LCLS, and I am looking forward to an upcoming beam time at LCLS. I view these recent experiments just as a beginning. We have demonstrated proof of principle for this new type of data collection, and now it is time to start making a serious impact on the field of structural biology of GPCRs and other challenging membrane proteins and complexes.
What are some of the most important types and features of G-protein coupled receptors that remain unsolved?
CHEREZOV: There is still a lot unknown about GPCRs. Although there were several significant breakthroughs made in the last few years, we are just scratching the surface. There are about 800 GPCRs in the human genome. Currently, only 19 structures of unique receptors are solved, and very little information is known about how GPCRs interact with their soluble signaling partners.
What additional advances do you hope to see in X-ray free-electron laser technology that could improve your results?
CHEREZOV: X-ray free-electron laser technology should allow researchers to look at more challenging receptors and complexes, for which only small microcrystals are available. Increasing the brightness of XFEL beams and improving detectors will allow us to look at even smaller crystals, approaching single molecules, as well as to begin to study their active behavior in real time.
What advances do you expect to see in the next decade of GPCR research?
CHEREZOV: The pace of structural studies in the GPCR field is breathtaking. We will understand most structural details of interactions between GPCRs and their partners. Structure-based drug design with GPCRs will become a reality. Hopefully, XFEL sources will make a major contribution to these advancements.
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.